MediBIC Group is aiming to deliver a health care platform optimized for each individual as a part of social infrastructure utilizing various approaches.
Gene Analysis & Banking
Genetic Testing Service
Knowledge of the genetic information of an individual can predict a person's risk for diseases and responsiveness to drugs prescribed by their doctor. We have developed and spread our genetic testing services with the intention of providing doctors with the diagnostic tools to routinely check a patient’s genetic information before prescribing a drug.
GLP-compliant Gene Profiling Service

In response to the growing number of requests for making the Genetic Testing Service available not only to consumers, but also to pharmaceutical companies for their clinical samples under our PGx clinical trial support service, we now offer a GLP-compliant gene profiling service.
Sample Banking & Management Service
Our DNA and cell banking service started as a joint project with the Foundation for Biomedical Research Innovation (BRI), a public interest incorporated foundation located in Kobe City. Handling clinical trial samples requires highly advanced and accurate quality control skills. For this reason, we obtained ISO9001 certification in February 2008. As MediBIC is the only company in Japan which provides sample banking services under a strict quality management system, our service has been receiving increased recognition. With the increasing prevalence of regenerative medicine, the need for storing cell and serum is growing. Also, MediBIC will expand our optional services beyond ample banking itself.
DNA information requires the highest possible security level in order to safely and correctly store and preserve personal information. Sample anonymization is mandatory. Not only single-coded, but double-coded anonymization is required if the samples are handled by multiple hospitals or companies. A dedicated management system (anonymization & sample management) is needed to exchange complex information accurately and quickly. MediBIC is the only company in Japan which offers this system.

Regenerative Medicine
Licensing

In recent years, many pet owners have begun to consider their pets as part of the family, and want their pets to receive state-of-the-art health care services as humans do. Many academic papers have been published emphasizing the similarity in pathology and pathological tissue that humans and dogs share. MediBIC Group utilizes data derived from treatment of spontaneous disease animals instead of using disease-induced lab animals. We aim to establish treatment and shorten the time between basic research discovery and clinical application in the field of regenerative medicine. In addition, we are planning to start a contract research service, leveraging the benefit of spontaneous disease animals and our broad network of clinical trial facilities in Japan (420 veterinary hospitals).
Cell Culture Systems
One of the critical elements in the attempt to industrialize regenerative medicine is the development of the apacity to grow cells with consistent yield and high quality. MediBIC Group has reviewed the whole culture process including stem cell characteristics and growth environment, and is currently developing a cell culture system through the new approach of “robotization of cell growth” instead of that of “robotization of human work”.
Consumables Sale
Along with the increased prevalence of regenerative medicine, it is expected that competitive or similar products will come to the market. We will build a patent strategy in the consumables field as well, to achieve mid- to long-term growth.
Big Data Analysis
Data Analysis for Preventive Health Care
MediBIC has accumulated expertise in the field of bioinformatics as applied to the factors that link “gene and medicine” or “gene and disease” as exemplified by our biomarker discovery business. Our goal is to prevent diseases before onset, and to reduce health care costs in the end.
In order to apply and further strengthen this technology, we believe that multilateral information analysis is required to integrate the enormous amount of ever-increasing health, medical and research data. Utilizing HP IDOL of Hewlett-Packard Japan, Ltd., the world’s most advanced technology with a track record of implementation, MediBIC will develop and provide a data analysis service which specializes in preventive medicine,
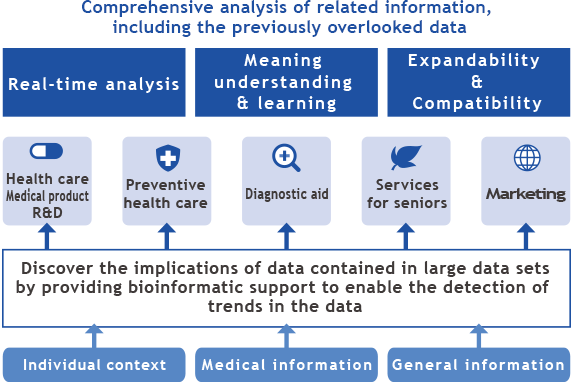
In the medical field overseas, a diagnostic aid system is available which presents diagnostic results automatically based on patient interviews.
Utilizing this leading-edge technology, we will develop a system unique to Japan, specialized to the medical field, which can provide preventive health care services in various ways.
Other Business
Drug Development
MediBIC Pharma is in charge of drug development (through its licensing business). Site Quality handles clinical trial support services(through its Site Management Organization business).
MediBIC Pharma is promoting licensing activities for the pancreatic cancer drug Glufosfamide.
Eleison Pharmaceuticals, Inc., in the US, initiated Phase 3 clinical trials for this anti-cancer agent in the US in October 2013, aiming for new drug approval in 2015. We were granted preferential negotiating rights and co-development rights for Glufosfamide in Asia. There is currently no plan to conduct clinical trials for Glufosfamide in Japan, but we will continue our business activities aimed at marketing the development and commercialization rights to pharmaceutical companies in Japan and other Asian countries.





